WHAT IS IT?
-----------
This model shows, graphically, the effect of depleting the Earth's ozone layer,
allowing more infrared radiation to penetrate the atmosphere and damage living
things.
DESCRIPTION OF THE MODEL
--------------------------
For the purpose of this discussion we will assume that turtles, quanta and
molecules are synonyms.
There are four breeds of turtles: ozone, chlorine, CFC and gamma. Each breed
carries a variable called age. Each patch stores a variable called
background-color whose purpose will be explained later. The main graphic
subroutine called "paint" draws a factory with three smokestacks and some trees
and grass.
Each smokestack can be switched on to emit CFC-laden smoke (gray turtles) or
switched off.
The ozone layer is initially represented by four hundred ozone molecules (red
turtles) moving randomly within a layer at the top of the screen.
The CFC molecules always move up towards the ozone layer. As soon as a CFC
molecule reaches the ozone layer it changes breed to chlorine and starts moving
in the same way the ozone molecules do. Thus chlorine molecules have the same
gray color as CFC molecules because only tne breed is changed. Chlorine turtles
kill ozone turtles i.e. if there are chlorine turtles on the same patch as the
ozone turtle, the ozone turtle dies. The chlorine turtles are also the only
turtles which age. As soon as the age of the chlorine turtle exceeds fifty the
turtle dies. We also create an ozone molecule with xcor set to 0 one at a time
as long as the overall number of ozone molecules is less than four hundred.
Gamma turtles which are created randomly in the upper portion of the screen
(above the ozone layer) always move down. If a gamma turtle collides with an
ozone turtle in the ozone layer the gamma turtle dies. However if the gamma
turtles makes it through the ozone layer it resets the color of the green patch
it lands upon to the background-color value; the visual effect is that of the
trees losing foilage and grass turning brown.
CHEMISTRY OF THE OZONE LAYER
-----------------------------
If you run this model you will see red turtles (ozone) "absorbing" pink turtles
(UV radiation) and a factory with three smokestacks that emit gray turtles (CFC)
into the ozone layer. The model is set up in such a way that it appears that CFC
destroy ozone which protects the green patches (the trees and the grass) from
ultraviolet radiation. However the interaction between the gray and the red
turtles in this model is very far from being the same as that between ozone and
CFC in the upper layers of atmosphere. Today scientists believe that it is
chlorine that causes mayhem in the ozone layer and CFC (acronym for
carbo-fluoro-chlorines) act as a transporting mechanism. A typical CFC --
CF(2)Cl(2) is a stable gaseous compound that easily gets into the ozone layer
without being depleted or oxidized in the lower layers of atmosphere. Once this
molecule gets into the ozone layer two atoms of chlorine break away from the
carbon skeleton as radicals and react with ozone forming ClO and oxygen.
Chlorine oxide further reacts with ozone forming chlorine radical and oxygen.
Thus chlorine radicals act as a catalyst that converts ozone into oxygen. Let
CF(2)Cl(2) denote the molecule of CFC, O(2) and O(3) denote oxygen and ozone
respectively and let ClO stand for chlorine oxide and let the asterisk * indicate
a free electron. Then the reactions that take place in the ozone layer can be
written as follows:
CF(2)Cl(2) ---------> CF(2)** + 2Cl*
O(3) + Cl* ---------> O(2) + ClO
ClO + O(3) ---------> Cl* + O(2)
It is not clear what the role of CF(2)** is and how Cl* eventually leaves the
ozone layer.
In this model we assume that the ozone layer has fixed capacity (four hundred
molecules) and that it has the ability to slowly regenerate itself. We also
assume that chlorine molecules literally "kill" ozone molecules which are on the
same patch and that chlorine molecules can live only up to a certain age (fifty)
so that we do not have accumulation of chlorine turtles. We do not even bother
to create ClO turtles and to set up the reactions because it will slow down the
performance of the program while still not giving accurate description of the
ozone layer.
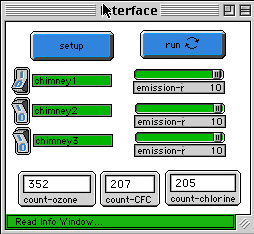
HOW TO USE IT
--------------
The only thing that user has control over in this model is the rate of emission
of CFC. You can turn either of the smokestacks on or off and you can change the
emission rate of each of the smokestacks with the appropriate slider. There is
only one plot-window that shows the concentration of ozone in the ozone layer as
a function of time. There are also three monitors that show the number of ozone,
CFC and chlorine molecules.
EXTENDING THE MODEL
-------------
If playing with the sliders controlling the rate of emission of CFC is not
enough, try to modify the source code so that green patches regenerate themselfs
that is if a green patch turns brown as a result of interaction with a gamma
turtle make it turn back to green after say 3 seconds.
STARLOGO FEATURES
-------------------
The purpose of this model is to demonstrate graphic capabilities of StarLogoT
rather than to explain the complex chemistry of the ozone layer.
Notice the use of breeds. One really has to introduce several breeds in order for
the molecules to move in the right way: the main purpose of chlorine breed is to
create a visual effect of CFC reaching the ozone layer and mixing with ozone.
Also it is important to use create-custom-turtles primitive in emit-CFC and
emit-gamma procedures, in this way one can keep track of newly created agents and
avoid reinitialization of gammas and CFC that were created at the previous step
of execution of the program.
REFERENCES
------------
Any standard textbook on Organic Chemistry usually contains a section on the
ozone layer andd CFC.
