Connected ChemistryÐ
Incorporating Interactive Simulations into the Chemistry
Classroom
Mike Stieff[1]
Uri Wilensky1
To appear in Journal of Science Education and
Technology
Please do not circulate or quote without authors'
permission
Suggested Running Head: Connected Chemistry
Corresponding Author's Mailing Address:
Mike
Stieff
2120 Campus Drive/Rm. 318
Northwestern
University
Evanston,
IL 60208
ABSTRACT
The aim of this article
is to describe a novel modeling and simulation package, Connected Chemistry,
and assess its impact on students' understanding of chemistry. Connected
Chemistry was implemented inside the NetLogo modeling environment. Its design
goal is to present a variety of chemistry concepts from the perspective of
"emergent phenomena"-- that is, how macro-level patterns in chemistry result
from the interactions of many molecules on a submicro-level. The Connected
Chemistry modeling environment provides students with the opportunity to
observe and explore these interactions in a simulated environment that enables
them to develop a deeper understanding of chemistry concepts and processes in
both the classroom and laboratory. Here, we present the conceptual foundations
of instruction using Connected Chemistry and the results of a small study that explored
its potential benefits. A three-part, 90-minute interview was administered to
six undergraduate science majors regarding the concept of chemical equilibrium.
Several commonly reported misconceptions about chemical equilibrium arose
during the interview. Prior to their interaction with Connected Chemistry,
students relied on memorized facts to explain chemical equilibrium and rigid
procedures to solve chemical equilibrium problems. Using Connected Chemistry
students employed problem solving techniques characterized by stronger attempts
at conceptual understanding and logical reasoning.
KEYWORDS: Chemistry Education, Modeling Environments, Scientific
Visualization
INTRODUCTION
Many
students view chemistry as one of the most difficult subjects to study at all
levels of schooling. Learning chemistry places many demands on students and
teachers that can seem insurmountable. Instructors display mathematical
formulas, chemical symbols and scientific measurements simultaneously to
describe phenomena that are not readily apparent to students. Moreover, the
concepts of chemistry are often seen as abstractions confined to the chemistry
classroom and not applicable outside of school. To deal with such difficulties,
chemistry educators have devoted considerable time to developing curricula that
help students visualize the molecular world and connect classroom concepts to
observable phenomena. In particular, a few novel curricula employ
computer-based learning environments, such as 4M:Chem (Kozma et al., 1996) and eChem
(Wu, Krajcik & Soloway, 2001), as visualization tools with which students
reason about chemistry. Building on these previous efforts, this paper
introduces a new computer-based modeling environment for learning chemistry.
The modeling environment, Connected Chemistry, uses a "glass box" approach
(Wilensky, 1999a) that not only enables students to visualize the molecular
world but also provides them with virtually unlimited opportunities to interact
with and to manipulate a simulated molecular world to gain a deeper
understanding of core chemistry concepts and phenomena. Here, we present the
conceptual underpinnings and structure of Connected Chemistry and some findings
regarding the affordances of using Connected Chemistry to learn about the
concept of chemical equilibrium.
Conceptual Difficulties in Chemistry Education
Considerable research has been devoted to identifying
and classifying student misconceptions in chemistry. The majority of this work
attempts to highlight where student understanding differs from accepted
scientific concepts in order to aid instructors in the development of new
curricula that bring students more rapidly to a desired understanding (Qu’lez-Pardo & Solaz-PortolŽs, 1995; Tyson &
Treagust, 1999; Voska & Heikkinen, 2000). To this end, educational
researchers have underscored how traditional chemistry curricula, replete with
lectures and drill-and-practice exercises, are unsuccessful at providing
students with a solid conceptual understanding of the theories and expressions
found in chemistry (Kozma et al., 1990; Johnstone, 1993; Thomas & Schwarz,
1998). Unfortunately, despite decades of research and curriculum development,
modern students still do not adequately learn the necessary concepts to succeed
in chemistry (Nakleh, 1992; Tyson & Treagust, 1999).
Some current researchers have begun to look beyond the
classification of misconceptions toward understanding what underlies the
difficulties that students have when approaching complex topics such as
chemical equilibrium, molecular orbital theory or reaction kinetics. In
particular, chemistry education research has shifted focus to explore students'
particular difficulties with understanding the representations of chemical phenomena at multiple levels as well as the forms chemists give those representations. Experienced
chemists take for granted that chemical phenomena occur at multiple levels --
the submicroscopic, the macroscopic and the symbolic (Johnstone, 1993). Because
interactions between molecules and atoms occur at a submicroscopic level,
chemists must refer to the objects and processes within their domain, which
they cannot observe directly, at a symbolic level. Moreover, aggregations of
molecules result in phenomena at a macroscopic level, which students can
directly observe, such as when water freezes or ice melts. Here again, for the
purposes of clarity and concision, chemists represent these macroscopic
phenomena at the symbolic level. At this level, where most teaching and
learning take place in the traditional chemistry classroom, instructors can use
multiple representations to describe the same phenomena (Kozma & Russell,
1997). A particular chemical reaction may be represented with letters,
molecular diagrams or plots of concentration over time. In the laboratory, students
are further expected to connect the symbolic representations in texts to the
macroscopic physical substances they use in an experiment and the numerical
measurements they take from laboratory instruments. Although chemists may
easily discern the relationships between chemical phenomena at the symbolic,
submicroscopic, and macroscopic levels and represent the phenomena with several
representations, students have considerably more difficulty (Banerjee, 1995).
One curriculum design approach that has enjoyed much
success in directly addressing the conceptual difficulties of chemistry
utilizes computer animations of chemical reactions to highlight the
relationships between the various representations and levels (Kozma et al.,
1996). These computer-based curricula provide an opportunity for inquiry-based
approaches to teaching chemistry. Curricula of this nature provide students
with a learning environment in which they can study a concept as it relates to
a particular system, be it a chemical reaction or an evolutionary ladder, make
predictions about that system, and justify their predictions with observable
outcomes (Barowy & Roberts, 1999). This approach discourages rote
memorization and algorithmic problem solving, while encouraging conceptual
understanding and critical thinking during problem solving (Garnett &
Kenneth, 1988). The inquiry approach has seen much success in many science
domains, and many chemistry educators now advocate for its use in the chemistry
classroom (Bodner, 1992; Copolo & Hounshell, 1995).
In the chemistry classroom, computer-based learning
environments attempt to make explicit the information embedded in traditional
molecular representations as well as to provide a visual representation of
molecular interactions for students to observe. Such software provides the
student with "multiple, linked representations" that foster conceptual
understanding and emphasize the connections between symbolic representations to
aid problem solving (Kozma et al., 1996. p.
41). Here students learn chemistry by viewing molecular animations side-by-side
with graphical outputs and chemical formulas. This is in stark contrast to
traditional chemistry lectures that almost exclusively rely on verbal
explanations of concepts in which students have no opportunity to observe
molecular interactions. Because, in the computer-based environment, students
can manipulate various parameters of the chemical reaction under study, they
can make predictions based on their understanding of relevant chemistry
concepts. Consequently, students can monitor their own learning by observing
the results of their manipulations of system variables.
Teaching Chemistry Concepts as Emergent Phenomena
Though computer-based learning environments for
chemistry have resulted in improved student understanding (Wu, Krajcik, &
Soloway, 2001), much of the current software is "first generation" and
does not yet fully employ an inquiry approach to teaching chemistry. Primarily,
much of the software currently available is limited by "black-box" designs.
These packages come with a predefined number of models, a small number of
activities for teachers and minor learning goals for students. Students are
given a limited number of macroscopic variables, which they can only manipulate
in predefined ways. Moreover, the molecular visualizations that give students
access to submicroscopic interactions between molecules are often delivered as
simple animations: for each set of variables that a student selects, one
particular animation repeatedly displays. These characteristics both reduce the
fidelity of these environments as simulations of actual chemical phenomena and
limit their use as inquiry tools with which students can make hypotheses and
explore outcomes.
To overcome these limitations, we suggest that a
better approach to the design of computer-based learning environments for
chemistry rests on the idea of teaching chemistry from the perspective of
emergent phenomena. The concept of emergent phenomena recognizes that patterns
observed on a macro-level "emerge" from the interactions between many agents on
a micro-level according to specific rules that govern individual micro-level
agents' behavior (Wilensky, 2000, 2001).
For instance, the specific submicroscopic interactions between many
billions of molecules in a drop of water result in the macroscopic physical
state of the water. When the water molecules move at relatively high velocities
and their interactions are elastic collisions, steam is observed on the
macro-level. Conversely, when the water molecules do not move, but vibrate in
place, and maintain contact with each other through strong hydrogen-bonding
interactions, the observer sees solid ice. Though these examples are
commonplace, our chemistry curricula have not been designed to let students
link the "rules" at the micro-level with their everyday experience at the
macro-level. In Connected Chemistry, we give students access to the rules that
govern the individual behavior of molecules. Students can then visualize and
explore how macro-level theories and concepts of the classroom emerge from
molecular interactions on the submicroscopic level. We believe that learning
chemistry from this perspective will both enhance students' understanding of
particular chemistry concepts and improve their problem solving skills across a
variety of chemistry topics. By conceiving of chemistry concepts as emergent
phenomena, students will gain additional avenues for reasoning about chemistry
beyond simple application of rote procedures and memorized facts. Next, we
present the modeling environment, Connected Chemistry, specifically designed to
teach a variety of chemistry concepts as emergent phenomena in both secondary
and undergraduate classrooms.
THE LEARNING ENVIRONMENT: CONNECTED CHEMISTRY
Connected Chemistry consists of a number of
computer-based models written in the multi-agent modeling language (MAML)
NetLogo (Wilensky, 1999b). The NetLogo language has been employed in a variety
of biology and physics classrooms (e.g. Wilensky & Reisman, 1999, in press;
Wilensky et al., 1999) to help students understand how micro-level interactions
between individual agents can result in observable patterns at the macro-level.
Frequently, in a traditional classroom, these observable patterns are taught as
isolated concepts and laws that students are required to memorize (Wilensky,
2000). In MAML environments, students can control the behavior of thousands of
graphical "agents". By exploring the relationship between the agents' rules of
behavior and the patterns that emerge as a result of these rules, students are
able to eliminate many misconceptions that are generated by confusing their
understandings of micro- and macro-level interactions. Typically, in curricula
using multi-agent modeling (e.g., GasLab (Wilensky, 1999a), EACH (Centola et
al., 2000)), students begin by exploring the behavior of pre-built simulations
designed to focus on some target concepts. They make predictions about the
behavior of the model under varying model parameters then test their
predictions by exploring model outcomes as they manipulate variables in a
simple graphical user interface. Students, however, may at any time open up the
"black box" of the interface and examine (and change) the underlying rules that
control the individual elements of the model. The Connected Chemistry package
consists of several such pre-built models[2]
designed for teaching target chemistry concepts. Each of the models simulates a
closed chemical system that students can interact with in several ways.
The core of every Connected Chemistry model is the interface
window (Figure 1). Typically, the
interface contains a graphics window,
a plotting window and several
variables in the form of sliders and buttons that students can manipulate. It
is here in the interface window that students can observe directly the
interaction between the submicro- and macro-levels of chemistry. The graphics
window is where students can observe
a visual representation of the interactions between simulated molecules in a
sealed container. The motion and interactions of the molecules in the graphics
window result from all of the molecules executing, in parallel, the individual
rules that govern their behavior; it is not a static animation linked to
manufactured states of the reaction. For instance, when a student alters the
temperature of the system, each molecule individual responds to the temperature
change-it is not a simple transition to a separate animation. Rather than
merely displaying a new animation of molecular motion at a higher temperature,
each molecule changes its behavior as if heat has been applied to the
boundaries of the closed system. When a molecule collides with the heated
boundary, it absorbs some quantum of energy. Consequently, each molecule that
collides with the boundary increases its speed in proportion to the amount of
energy it absorbs, independent of the other molecules in the system. Thus, the
primary affordance of the graphics window is that students can observe directly
how changes in macroscopic variables (e.g. adjustments to temperature,
concentration, etc.), which they
make, affect the submicroscopic interactions of the molecular species that, in
turn, perturb macroscopic graphs of variables such as concentration, pH or
pressure.
So that students can observe the effects of their
manipulations of the system on macroscopic variables, each interface window
includes at least one plotting window. A basic plotting window in a Connected
Chemistry model typically displays the relative concentrations of each chemical
substance in the system in real-time as the simulation runs. The concentration
of a substance is a macro-level quantity of measurement that is defined in
Connected
Chemistry by the number of molecules of a particular
chemical species relative to the total number of molecules in the system, which
parallels the macroscopic concept of molarity (i.e. moles of a dissolved
substance relative to the total volume of a solution). Once the simulation
begins, the system quickly reaches a steady-state equilibrium, which students
can observe (and perturb) in both the graphics window and simultaneously in the
concentration plot. Were the model confined to demonstrating the properties of
the system in either window alone, it would be severely limited. A fundamental
asset of Connected Chemistry as a learning tool for chemistry comes from the
fact that students can interact with the system to modify system parameters and
receive instant feedback about their predictions with multiple representations
on multiple levels. Many Connected Chemistry models contain additional plot
windows for students to observe a variety of phenomena such as changes in pH
over time, changes in pressure due to concentration and changes in
concentration of a reaction product due to changes in the amount of added
catalyst.
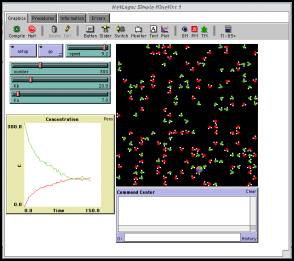
Figure 1. Connected
Chemistry's interface window contains a graphics display, plot outputs and
system parameters.
For instance, in the "Simple Kinetics 1" model (shown
in Figure 1), students can observe in the graphics window how a collision
between two nitrogen dioxide molecules, under favorable conditions, can produce
one dinitrogen tetroxide molecule. As a result, the macro-level representations
of the chemical species change. In the graphics window there is an increase in
the number of dinitrogen tetroxide molecules, and in the plotting window, the
concentration plot of dinitrogen tetroxide also increases while that of
dinitrogen monoxide decreases. By manipulating the sliders for predefined
system variables in "Simple Kinetics 1", students can also observe how varying
the rate constants for the reactions alters molecular behavior and consequently
the concentration plot. Connected Chemistry models are not limited to the
predefined variables of the software designers, however. The models are
extremely malleable: Students can add or remove variables from the interface
window to explore thoroughly the behavior of a chemical system under a variety
of conditions.
As mentioned
previously, a critical advantage of Connected Chemistry over other
computer-based inquiry environments is that each model is built as a
"glass-box". Each model contains an information window (Figure 2) that contains a description of the
chemistry concepts underlying the model, instructions on how to use the
interface window and some suggested modifications of the procedures. As
described earlier, students can manipulate the variables that are provided in
the interface window and explore the resultant model behavior. But Connected
Chemistry also enables students to go one level deeper. In the procedures
window (Figure 3) they can also
examine and alter the NetLogo programming code that governs molecular behavior.
The NetLogo code is easily accessible and comprehensible, which enables
students to "read" the rules that the molecules obey and, if they wish, to
later modify them. The Connected Chemistry documentation encourages instructors
and students to modify procedures and observe how the chemical system is
affected. For example, students might alter the procedures so that they can
inject more chemical reactants or products into the system as the simulation
runs.
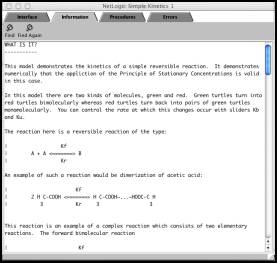
Figure 2. The information window of each Connected
Chemistry model contains a summary of the chemistry concepts highlighted by the
model as well as instruction on how to use the model and suggestions on how to
manipulate the NetLogo code to further explore the simulated chemical system.
Though at first it
may seem daunting to alter the NetLogo code, each model comes with several
supports for students and teachers. First, each Connected Chemistry model comes
packaged with an on-line tutorial and user-manual for the NetLogo modeling
language. NetLogo stresses natural language programming, and using the on-line
tutorial many students are able to design novel models in less than a week.
Second, the procedures window
(Figure 3) of each Connected Chemistry model is immediately accessible from the
interface window so that students can investigate the procedures that underlie
the visual displays of the graphics and plotting windows. Moreover, the
procedures listed in the procedures window are fully annotated with concise
comments that explain how the particular code used results in the observed
behavior of the interface window. Third, the information window of each Connected
Chemistry model contains a section entitled "Extending The Model" that provides
suggestions to students about how they might alter the code to further explore
the chemistry concepts emphasized by the model. The immediate access to and
straightforward language of the underlying procedures provides a significant
advantage to chemistry students. This glass-box design of Connected Chemistry
allows students to explore concepts outside the initial boundaries of each
model. Thus, novel problems and spontaneous questions can easily be addressed
as they occur in the classroom. With minor alterations of the interface window
or procedures, students can introduce new variables into a model to test their
predictions. For example, students working with the "Simple Kinetics 1" model
may decide to alter the procedures so that three molecules of nitrogen monoxide
must collide in order to form one molecule of dinitrogen tetroxide. After
making their modifications to the procedure window, the students can then
observe how their alterations affect the rate of the reaction and the
concentration of each chemical species over time. Working with their instructor
the students might further discuss the differences between bimolecular and
trimolecular reactions and whether the students' novel chemical system could
actually occur in the real-world given their knowledge of kinetics (and
stoichiometry). Together the procedures window, the interface window and the
information window draw together the submicro-, macro- and symbolic levels of
chemistry so that students are not forced to rely on only one level or
representation.
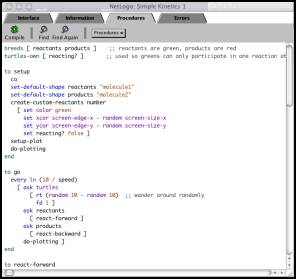
Figure
3. In the procedures window, each Connected Chemistry model provides students
with access to the code that drives the simulation. The NetLogo modeling language
of Connected Chemistry is uncomplicated, and annotations in the procedure
window explicitly explain the purpose of each procedure to students.
POTENTIAL BENEFITS OF USING CONNECTED CHEMISTRY
To explore the benefits of the Connected Chemistry
learning environment for helping students understand chemistry, we conducted a
small study with six undergraduate students at a large research university. In
particular, we were interested in observing whether Connected Chemistry
provoked changes in students' understanding of the concept of chemical
equilibrium. Although Connected Chemistry is designed primarily for use in an
introductory chemistry course at the secondary level, we conducted the current
study with undergraduate science students in order to measure the success of
the software with students well versed in the concept of chemical equilibrium.
While it is true that these six students might possess both more domain
knowledge and more advanced problem-solving skills than secondary students,
subsequent ongoing work with secondary students suggests that Connected
Chemistry possesses the same opportunities for both populations. Our goal here
was to observe whether Connected Chemistry provided significant benefits to the
advanced students, which would warrant a larger implementation study at the
secondary level.
Study Participants
Each of the students interviewed in this study was
self-described as high achieving in the sciences, particularly chemistry and
biology. Of the six undergraduates, two were fourth-year students who intended
to pursue doctoral work in biochemistry and molecular biology. Of the other
four students, all of whom intended to pursue degrees in medicine, two were
third-year students and two were fourth-year students. All six of the students
reported originally learning about chemical equilibrium in introductory high
school chemistry and that they had not covered the concept again explicitly in
any of their higher-level chemistry courses. Each student had successful
completed undergraduate courses in General Chemistry, Organic Chemistry, and
Biochemistry with a greater than B average. Before the start of the interview,
each student claimed to be familiar with the concept of chemical equilibrium.
Study Protocol
The
protocol for the study consisted of three separate segments in a 90-minute
interview (see Table I; for a full outline of the interview protocol, please
see Appendix I). The first segment of the interview aimed to uncover each
participant's recall and understanding of chemical equilibrium. For this,
participants were asked various questions that allowed them to explain, in
their own words, chemical equilibrium and any information they felt was
important to the concept. The second portion of the interview attempted to
discover each participant's ability to apply chemical equilibrium concepts to
traditional textbook questions. The final portion of the interview protocol
required students to make predictions about the equilibrium state and position
of a particular reaction, 2NO2§ˆN2O4, which they then demonstrated using the Connected
Chemistry model, "Chemical Equilibrium 1". For each question in the final
segment of the interview, participants were given an initial set of conditions
for the system followed by a final set of conditions in which one or two system
parameters were changed while the others remained constant. The simulation was
first run using the set of initial conditions and participants would observe
the molecular interactions. The interviewer would then pose a question about
how the system would change if the system variables were altered and the
participants would make predictions. The participants then manipulated the
system variables as defined by the interviewer's question, after which they
would explain their observations of the system and compare them with their
initial predictions. The first two segments together comprised 45 minutes of
the interview, and the third segment comprised the remaining 45 minutes.
Table I. Format of Protocol Questions
|
Interview Segment |
Example Questions |
|
Recall and Explain Chemical Equilibrium |
"Define chemical equilibrium." "State factors that can affect reaction
equilibrium." |
|
Traditional Problem Solving |
Ag2CO3(aq) +
2HCl(aq)<--->2AgCl(s) + H2CO3(aq) "Do the states of each of these substances have any
bearing on the equilibrium position of the reaction?" |
|
Problem Solving in Connected Chemistry |
Chemical Equilibrium 1 models 2NO2 (g) <---> N2O4
(g) "Can you tell by looking at the graphics window only
if the system is in equilibrium?" "What else would you need to know?" "What will happen if you decrease the volume of the
container?ÉYou decreased it, what happened to the system? Is this what you
predicted?" |
Why Model Chemical Equilibrium?
Before detailing the results of our study, it may be helpful
to first elaborate on why we chose chemical equilibrium as one of the first
concepts to model in Connected Chemistry. The concept of chemical equilibrium
and its' related ideas is frequently introduced to students in a high school
general chemistry course, where it is often the first and last chemistry course
in which the concept is directly addressed (Voska & Heikkinen, 2000).
Though chemical equilibrium is rarely taught in higher-level courses, it is a
recurrent concept that is vital to understanding many chemical processes not
only in chemistry but also biology, medicine and engineering. In fact, chemical
equilibrium plays a central role in oxidation-reduction reactions, acid/base
behavior, the design of pharmaceuticals and biochemical interactions that
underlie many biological processes (Qu’lez-Pardo & Solaz-PortolŽs, 1995).
Though experienced chemists are so familiar with this concept that they rarely
acknowledge it explicitly, educational researchers have found that many
students and instructors often find the concept and its accompanying principles
difficult to master (Van Driel et al., 1999; Tyson
& Treagust, 1999; Banerjee, 1991). Because of the difficulty
students have in mastering the concept, there is a critical need for chemistry
educators to develop novel curricula that facilitate a better understanding of
chemical equilibrium.
At its
most basic level, the concept of chemical equilibrium requires that students
understand the relationships between several physical variables (e.g.,
pressure, temperature, concentration), several mathematical and symbolic
expressions (e.g., reaction quotient, equilibrium constant, rate law), and the
equilibrium position (i.e. the relative ratio of reactants and products) of the
chemical reaction in question. Typically, students learn the concept of
equilibrium by memorizing LeChatelier's Principle, a list of rules for
predicting the equilibrium position of a chemical reaction based on the values
of the aforementioned physical variables (Tyson & Treagust, 1999; Appendix
II contains a summarized version of LeChatelier's Principle). As with many
other scientific concepts, the traditional emphasis on fact memorization and
rote procedures often results in poor understanding, retention and application
of chemical equilibrium concepts (Qu’lez-Pardo & Solaz-PortolŽs, 1995).
Results
Observing the participants' interactions with
Connected Chemistry revealed several common misconceptions about chemical
equilibrium and incorrect applications of LeChatelier's Principle. Though these
misconceptions served as a point of reference from which we observed changes in
student thinking and problem solving, we will not detail them here as they have
been reported at length elsewhere (Banerjee, 1991; Qu’lez-Pardo
& Solaz-PortolŽs, 1995). Rather, our observations of student
thinking over the course of the interview, most notably during the use of
Connected Chemistry, centered on a dramatic change in conception, articulation,
and problem solving. Most important, we observed that the participants'
dependence on rote procedures for problem-solving and fact memorization, which
governed their thinking in the first two stages of the interview, gave way to
more thorough attempts at conceptual reasoning and logical justification of
answers during their use of Connected Chemistry Ð a desired result of
inquiry-based curricula (Barowy & Roberts, 1999). Moreover, the students'
automatic acceptance of information from texts and lectures was replaced by a
willingness to question facts and theories in order to validate personally
their answers and observations in Connected Chemistry. Such changes in student
thinking were especially salient in three distinct categories: (1) defining
equilibrium for a chemical system, (2) characterizing factors affecting equilibrium,
and (3) transitioning between submicro-, macro- and symbolic levels during
problem solving.
Equilibrating Thinking about
Equilibrium
The most prominent effect we observed from
participants' interaction with Connected Chemistry was the marked clarifications
of the definition of chemical equilibrium that each student made. Difficulty in
defining equilibrium is often found among chemistry and biology students when
students misconceive of equilibrium as a static state Ð one in which no change
occurs (Banerjee, 1995; Kozma et al., 1990). When a chemical reaction is in
equilibrium, the rate of conversion of reactants to products is equal to the
rate of conversion of products back into reactants. Thus, at equilibrium the
relative concentrations of each molecular species remains constant on the
macro-level; however, on the submicro-level, individual molecules are
continually converted from reactant to product and back again. Chemical
reactions do not stop when they reach equilibrium. Thus, equilibrium is a
dynamic process, which many students fail to see because of the stable
concentrations of each molecular species. Surprisingly, the students involved
in our study had no initial difficulty understanding the dynamic nature of
equilibrium. When questioned during the first segment of the interview, each
participant stated that although the concentrations of chemical species
remained apparently constant, reactions continued on the submicro-level at
equilibrium. The following excerpt from the interview with Jane (all references
to participants use pseudonyms) typifies participant responses.
Interviewer: So,
we don't have to have to have the same amount of A and B [reactants] as we do C
and D [products] at equilibrium?
Jane: No.
Interviewer: So
what is the same at equilibrium?
Jane: The
reaction rate forward and backward.
Interviewer:
Is the concentration on
each side changing at equilibrium?
Jane: No,
it's staying the same.
Interviewer: Then is anything
changing at equilibrium?
Jane: Well,
yeah. Like some of A and B are going to be C and D and some of C and D are
going to be A and B, but the forward and backward [rate] are equal so it all
balances out and all the concentrations stay the same.
Though at first it appeared that each of our
participants possessed a stable, accurate definition of chemical equilibrium,
inconsistencies in their conceptual understanding quickly became apparent when
they were asked to describe the equilibrium state of specific chemical
reactions during the second segment of the interview. While answering questions
regarding an equilibrium position and how different variables would affect that
equilibrium, the students all had difficulty. Their previously solid
definitions and conceptualization of chemical equilibrium fluctuated to depend
sometimes on rate and sometimes on concentration. Their reasoning often failed
to include vital characteristics of the chemical species in question, such as
whether a product boiled out of solution as a vapor. Even when the interviewer
pointed out these characteristics, the students had difficulty incorporating
the information into their explanations, often declaring that the
characteristic was not important. One participant, Andrew, exemplified these
common confusions when attempting to predict in which direction Reaction 1
proceeded. Though he correctly answered the question by stating that
equilibrium is reached when the competing forward and reverse reaction rates
are equal, he became fixated on the macro-level concentrations of each chemical
species and incorrectly changed his answer to depend on those values; in doing
so, he completely ignored the role of reaction rate.
Ag2CO3 + 2HCl (aq)… 2AgCl
(s)+ H2CO3 (aq)
(Reaction 1)
Interviewer: Let's say this reaction is
frozen in time at specific molar concentrations . . . 10 [Ag2CO3],
10 [HCl], 5 [AgCl], and 5 [H2CO3]. If we start time
again, will the reaction change at all or will it stay this way?
Andrew: You're
going to get production of more product.
Interviewer: Why is that?
Andrew: Because
the molar concentration of Ag2CO3 and HCl are a lot
higher than what you have on the product side. So it is going to push the
reaction forward [to make the products AgCl and H2CO3].
Interviewer: So,
when would it stop or would it stop going this way [to the right]? É You said
it would push it this way, would it stop pushing this way ever or will it keep
going until all of these [reactants] are gone?
Andrew: Well
it would stop as soon as the rate of formation of products is equal to the rate
of dissociation of those products back to those reactants.
Interviewer: What
would happen if we started off with, instead of 5s [concentrations of both
reactants], with 10s [concentrations of both reactants and both products].
Would that change?
Andrew: Yeah-I
think-I don't think solids matter. You're only dealing with aqueous solutions.
I guess gases too, but in this case you don't have that. So in that case, you
have a total of, here we have 10 [Ag2CO3] and 10 [HCl]
and there you have 10 [H2CO3], so there is an imbalance.
Interviewer: Okay, let me balance
it then for you. So now we say that these 20 [H2CO3]
balance these 20 [reactants]Éwould that change?
Andrew: Well,
sticking to what I believe, that would stay the same.
Interviewer: What
if I told you that this solid [AgCl] was precipitating out of solution? It's
actually falling out of the aqueous solution into a solid on the bottom.
Andrew: I
don't think it's going to change anything.
From
Andrew's comments, it is apparent that his concept of chemical equilibrium was
not stable. Although he correctly identified that the reaction will reach
equilibrium when the forward and reverse rates are equal (an assertion he also
made earlier in the interview), he fell victim to the common misconception that
equilibrium occurs when the concentrations of chemical species on each side of
the double-headed arrow are equal. Andrew also attempted to draw on tangential
knowledge that a chemist would consider irrelevant and incorrect in this
context to justify his answer. For instance, he stated, "Solids don't matter."
Here, he seemed to rely on rote memorization to solve the problem. Andrew
correctly recalled a rule that applies when writing the mathematical
equilibrium expression; however, it does not apply to the problem in this
excerpt. If Andrew had been asked to define the equilibrium expression, a
mathematical representation of the equilibrium state, this rule would be
requisite.
Andrew's
dependence on rote memorization again failed him by causing him to neglect the
importance of precipitants in the chemical reaction. During the interview, he
failed to notice that the AgCl was precipitating out of solution in the
reaction. Because his neglect of this fact may be attributed to an oversight on
his part or difficulty in interpreting the symbolic representations of chemical
reactions, the interviewer made the information explicit. Despite the attention
that the interviewer gave to this point, Andrew indicated that it was not
relevant to the problem. A solid conceptual understanding of the nature of
chemical equilibrium would have allowed Andrew to realize that any means by
which one of the product species is removed from the solution decreases the
effective concentration of that species in the reaction. The subsequent application
of LeChatelier's Principle to this problem would result in the correct
prediction that the reaction would proceed forward and generate more products.
None of the students in our study was able to identify the important role of
precipitates (or evolved gases), though each student referenced a variety of
other facts and rules that they incorrectly believed affected the equilibrium
of a reaction.
Over the course of his 45-minute
interaction with Connected Chemistry, a transition in Andrew's articulation of
chemical equilibrium became increasingly apparent. Whereas Andrew initially
relied almost exclusively on rote procedures and memorized facts to answer
questions about chemical equilibrium, his problem-solving techniques took on
the more critical aspects of a scientific researcher while he was engaged with
Connected Chemistry. When discussing his reasoning during the third segment of
the interview, Andrew was able to recognize and correct his own misconceptions
about the definition of chemical equilibrium. By observing how the interactions
between molecules in the graphics window changed according to system variables,
Andrew identified, evaluated, and refined his understanding. As in other inquiry-based curricula,
Andrew's re-conceptualization of chemical equilibrium depended heavily on the
context of the Connected Chemistry modeling environment: the model allowed him
to make predictions and receive instant feedback and also provided
opportunities to validate and justify answers by exploring alternative paths of
reasoning.
The
nature of Andrew's change in reasoning became clear during the final segment of
the interview. At first, he continued to justify his predictions with rote
procedures, but as he received feedback that contradicted his predictions, he
articulated and resolved his misconceptions using more conceptually sound
arguments and logical reasoning. As he did in the paper-and-pencil exercises,
he first confused rate and concentration and decided that identical product
concentration and reactant concentration determines chemical equilibrium. After
running the simulation and observing that reactant concentration and product
concentration differ at equilibrium, his understanding was challenged and he
began to clarify whether rate or concentration determines equilibrium.
Interviewer: [Pointing
to the concentration plot.] Okay, so that means that the rate is constant
between them?
Andrew: Yeah,
so the amount of red [products] turning to green [reactants] and the amount of
green turning to red, that rate is equivalent, but somehow they're satisfied
with the fact that there is more green than there is red.
Interviewer: So
does that fit in with what you said earlier that we would make equal amounts of
greens and reds?
Andrew: No.
Interviewer: Can you explain why
that's happening?
Andrew: UmÉMaybe
it's because I overlooked the coefficients, you require two molecules of NO2
to N2O4 [see Reaction 1]. It's not a 1:1 ratio.
Interviewer: Oh,
so we would always have more greens than reds?
Andrew: Yes.
Because you're going to need a lot more greens to make a red as opposed to the
other way around. That's a basic concept I should've known.
Though Andrew used the model to refine his concept of
equilibrium, he did not immediately clarify his understanding. In the above
excerpt, Andrew indicates that there will always be a greater number of
reactants because of the "coefficients", which he based on the chemical
equation that states two NO2 molecules combine to form one N2O4
molecule. Here, he showed a dependence on the symbolic representation of the
reaction for his explanation. The benefits of Connected Chemistry to help
Andrew better clarify and articulate his understanding of chemical equilibrium
become apparent as his interaction progressed in the following excerpt. Here,
Andrew used Connected Chemistry to test his prediction that there will always
be more reactants than products at equilibrium, and by coordinating the
information in the plotting and graphics windows, he resolved his confusion.
Interviewer: So,
would we always have more greens than we have reds though? Could we have more
reds than greens?
Andrew: UmÉyeah.
Interviewer: Why?
Andrew: Because
at certain points when there's no more NO2 to react with each other
and you have a bunch of N2O4, it's going to go-want to
go-back and dissociate into greens. At that point you'll have more reds.
Interviewer: Okay,
but could we have a state that's at equilibrium where we have more reds than
greens. Like we have 5 greens and 20 reds? Those numbers are arbitrary, just
not 0 greens and more reds.
Andrew: I
would still say no, because it still requires two greens to make a red.
[Andrew adds more reactants
to the model.]
Interviewer: So now what do think is
going to happen?
Andrew: ÉSo
now we have a lot more of this (green) to begin with. Well, I am going to see a
similar trend, but up here [points to the top of the y axis]É I'm wondering
what will happen to the red, will it increase, but I don't think so. Should I
press run?
Interviewer: Go
aheadÉ So, you looked surprised, we've got more reds than greens. [Points to
the graphics and plotting windows.]
Andrew: Yeah,
I was afraid that was going to happen. [Laughs.]
Interviewer: Does that make any
sense? Would you say they are at equilibrium?
Andrew: Yeah.
So this obviously is what happens ideally. Like this is the correct way?
Interviewer: Yeah, no deception
here.
Andrew: Right.
Based on that this tells me that what I said was wrong before. I can conclude
from this that if you dump in a whole bunch of NO2 then you're going
to get an even higher proportion of N2O4 red at a certain
point. And you have so much more red that's going to dissociate back to green.
But because you initially added so much green you caused the increase to redÉ
Andrew's interaction with Connected Chemistry in the
interview typifies the support that Connected Chemistry can provide students
during problem solving. Because the student participants were able to use the
Connected Chemistry model to test their predictions and receive instant
feedback, they were able to evaluate and modify their individual understandings
of chemical equilibrium. The form of this feedback is not trivial. Connected
Chemistry models, such as the chemical equilibrium model used in this study, do
not simply tell students whether their predictions are right or wrong. Rather,
students must observe the behavior of the simulated chemical systems in
Connected Chemistry and decide if the state of the system matches their
predictions. As Andrew discovered, Connected Chemistry provides students with a
Ôvirtual laboratory' where they can make repeated alterations to the system and
observe the effects when their predictions fail to explain the behavior of the
system, Our choice of undergraduate participants revealed that even students
who are conversant with a chemistry concept can use Connected Chemistry
feedback to evaluate and refine their understanding.
Focusing on the Factors, not Just the Facts
In
addition to reinforcing participants' conceptualization of chemical
equilibrium, Connected Chemistry supported participants' reasoning about how
system factors could affect the equilibrium of a chemical reaction. In our
interview, we placed a heavy emphasis on the use of LeChatelier's Principle
when discussing equilibrium problems, although we are aware of the controversy
surrounding its use.[3]
When using LeChatelier's Principle, the participants displayed little-to-no
recall of the major system factors (i.e. pressure, concentration and
temperature) that the principle emphasizes. More notable was the students'
inability to describe the causal mechanisms for how each factor affected
chemical equilibrium. By far, the participants were unable to describe
conceptually the effects of any factor; instead, as we see in this excerpt with
Darren, they simply parroted the factors from the summarized list that we
provided them with during the interview.
Interviewer: You
said that concentration can affect the equilibrium of a reaction. Can you think
of anything else that might affect it?
Darren: YeahÉTemperature,
concentration, bond energyÉI'm not sure what else.
Interviewer: Of
those three things, can you say why temperature, concentration, or bond energy-
Darren: I
meant entropy.
Interviewer: Okay,
entropy, so what do those have to do with the equilibrium?
Darren: They're
all forces, I guess that are pushing on it in different directions. So if there
is a higher concentration on one side of the equation that's going to tend to
drive the reaction the other way. If there is higher entropy on one side, it's going
to drive the reaction in the other way. If there's higher temperature, it's
going toÉuhÉtemperature seems like it would drive it both ways.
Interviewer: Why
is that?
Darren: Just
that the higher temperature is going to make the reaction easier to happen. It
seems it would have the same effect both forward and backward.
From this excerpt, we see that Darren has a minimal
understanding of the conceptual basis for why each of the factors affects
equilibrium. Although he readily provided three factors, only two of which
(temperature and concentration) are included in LeChatelier's Principle, he was
unable to justify the causal mechanism behind each factor's influence. As with
the other students, Darren's attention to fact memorization was evident. He explained
that a variable that is greater for one side of a chemical equation would push
the reaction in the opposite direction. This is the standard format of the
principle found in textbooks (see Appendix II). His lack of conceptual
understanding caused him to apply this same reasoning to entropy, a factor that
he later underscored as a major determinant of the equilibrium state of a
chemical reaction. Unfortunately, entropy actually affects chemical equilibrium
in a manner opposite Darren' prediction: Reactions are driven toward the side of the equation that has a greater amount of
entropy.
Darren's
responses to the external factor questions are typical for high school and
undergraduate students who have had traditional lessons on chemical equilibrium
(Banerjee, 1995; Kozma et al., 1990). Though it is not the goal of the
Connected Chemistry curriculum to provide a thorough explanation behind an
external factor's influence via quantum physics, it does attempt to provide
students with a qualitative perspective on the causal mechanisms underlying
each factor. Connected Chemistry's fulfillment of that goal was evident in
Jane's interactions with the software. Though she was able to correctly
identify the three factors for which LeChatelier's Principle accounts, like
Darren, she was unable to justify conceptually why or how the factors affected
a reaction's equilibrium. Jane justified the effect of each factor by stating
that the factor affected equilibrium because "LeChatelier's Principle says
so". Despite Jane's lack of conceptual understanding, she was able to
successfully answer many of the questions from memory both on paper and using
Connected Chemistry. The underlying flaws in her thinking became apparent when
she attempted a more difficult problem in which she was asked to alter the
ratio of products to reactants in the simulation by adjusting the variables in
the interface window.
Interviewer: Do
you think it's possible that you could get the ratio of reactants to products
to be 2:1?
Jane: Yes.
Interviewer: Do
you think you could get them to maintain that ratio?
Jane: Yes.
Interviewer: How
would you do that?
Jane: By
changing the volume, you can get themÉ Like we saw earlier. If we decrease the
volume, suddenly we have more reds. So you could mess with the volume until you
got the right ratio.
Interviewer: Okay,
could you go ahead and try to do that ?
Jane:
Trying
to?
Interviewer: Trying
to get 2 greens [reactants] for every 1 red [product].
[Jane interacts with the model for a few moments.]
Interviewer: So
what have you done here?
Jane:
I
decreased the volume.
Interviewer: Right.
And is it moving closer to 2:1?
Jane:
UmÉno.
[She inspects the plotting window.] It's farther apart.
Interviewer: So,
is there something else you think you could do? Change the volume or change
something else?
Jane:
Change
the volume the other way maybe?
Interviewer: Okay,
do you want to try that?
[Jane increases the volume a few times, but is
unsuccessful at reaching the 2:1 ratio.]
Jane: NoÉ
Probably just changing the temperature then.
Interviewer: So
what effect would changing the temperature have?
Jane:
It
changes the Qc [in our case, the equilibrium constant].
Interviewer: Okay.
Jane:
So,
it would change the ratios.
Interviewer: Ah,
okay, so out of these things [points to system variables], which do you think
you could use to change that ratio?
Jane:
Temperature.
Interviewer: Just
the temperature?
Jane:
OH!
You could change the forward and reverse reaction rate!
Interviewer: Okay,
so they'll change that Qc has well?
Jane: Yeah.
Interviewer: So
manipulating those variables, you could get it to a point where they were 2:1?
Jane:
Yeah.
Interviewer: But
could you use either the concentrations or the volume or pressure?
Jane:
No.
Jane's
problem solving strategies at the start of the interview were based solely on
fact recall, and she successfully answered many of the interview questions from
memory. Though she was able to identify the three factors of LeChatelier's
Principle, she was unable to accurately describe the way in which each factor
affected the equilibrium of a system, let alone the reasons why each factor
even had an effect. A more solid conceptual understanding of the influence of
each factor would have deterred Jane from incorrectly adjusting the volume to
answer the problem. Through her interactions with the software she was able to
test her knowledge in the simulation. Connected Chemistry provided Jane with
the necessary feedback to determine why each factor affected the equilibrium in
addition to identifying the effects of other factors, such as rate constants,
not accounted for by LeChatelier's Principle. By observing how the equilibrium
changed in relation to her manipulation of a variable, she was able to reason
that only temperature or rate constants could alter the ratio of the two
chemical species. Modifying concentration or volume, which Jane first
attempted, resulted in different reactant and product concentrations, but the
ratio of the two remained the same. Although Jane rationalized her answer with
trial-and-error manipulations in our interview, we believe that students and
instructors can use Connected Chemistry more effectively in the classroom. When
using Connected Chemistry to learn about the concept of chemical equilibrium,
students have the opportunity to view the procedures window and explore the
rules that govern the molecules in the graphics window to see just how each
variable affects the chemical system. If they are unclear about the
interactions that they observe in the interface window, students can view the
comments in the procedures window that explain the molecular interactions of
the graphics window. Thus, Connected Chemistry has the potential to provide
students with the causal mechanisms behind each factor with both written
explanations and molecular visualizations that would not limit them to
recalling memorized facts that are easily forgotten or confused.
Putting Levels on the Playing Field
A
third manner in which Connected Chemistry benefited the participants was that
it allowed them to more explicitly observe the connections between the
submicroscopic, macroscopic and symbolic levels of chemistry. Besides the
inability to correctly recall memorized facts, it is possible to attribute many
of the difficulties that the participants had during problem solving to
confusion about these levels of description. The concept of emergent phenomena,
on which Connected Chemistry is based, emphasizes both recognizing levels and
transitioning between them to fully understand a concept and solve related
problems (Wilensky & Resnick, 1999). The inability of our participants to
accurately define chemical equilibrium or to understand how external factors
affect a reaction seem to stem partially from the participants' inability to
relate macro-level phenomena to events on the submicro-level. As mentioned
previously, this confusion is further exaggerated in students who have
difficulty relating these two levels to the representations on the symbolic
level required in chemistry. Examples of levels confusion were abundant in each
of the interviews. Brief excerpts from Mary and Kathy provide typical responses
when participants predicted the effects of adding a catalyst or an inert gas to
the system.
Interviewer: You
mentioned earlier something about adding an enzyme or a catalyst to the
reaction. What did you say that would do?
Mary: That
should increase the reaction rate.
Interviewer: Okay,
the rate going this way [forward] or this way [going backward].
Mary: Um,
I think the catalyst is specific for the direction.
Interviewer: So, if we were to add an
enzyme or a catalyst-an enzyme is a biological catalyst-
Mary: Yes.
Interviewer: -to a system and it
would favor either greens [reactants] going to reds [products] or reds going to
greens?
Mary: Right.
(Mary runs the
scenario in Connected Chemistry.)
Interviewer: What effect are they
[the catalyst molecules] having on the system? [She thinks for a while.] Is the
equilibrium changing at all?
Mary: [She
looks at the graphics window then the plotting window.] No.
Interviewer: Does
that make sense?
Mary: I
would have expected the catalyst to accelerate the reaction in one direction.
Interviewer: What
do you think it's doing?
Mary: Hmm...
Maybe it accelerates it in both directions.
*****
Interviewer: And if we don't change
the volume of a container at all, but let's say we pump in some Argon gas, an
inert gas, so that we increase the pressure of the container, does that change
the reaction in any way?
Kathy: I
think there will be more N2O4.
[Later, Kathy runs the simulation.]
Interviewer: Has adding the inert
gas changed the system? Did it upset the equilibrium of the system?
Kathy: No.
Interviewer: But that's not what you said
it would doÉ
Kathy: Yeah.
Interviewer: Can you say why not?
Kathy: HmmÉ[she
points to the graphics window] because it's not reacting with itÉThe inert gas
is not reacting-it's not supposed to, that's why it's inertÉ LeChatelier's
Principle would say that we increased the pressure and we would get more reds, but
obviously that's not happening. So maybe we need a caveat to the principle.
The
excerpts from these two participants reveal how Connected Chemistry can help
students understand the relationship between different levels in chemistry.
Both Mary and Kathy were able to use the explicit visualization of the
submicro-level molecular interactions displayed in the graphics window to
correct their misconceptions. Mary initially predicted that catalysts have a
unidirectional effect on a chemical reaction, which is not surprising given
that students often employ catalysts in the laboratory, at the macro-level, to
accelerate the formation of products. Kathy also makes an initial prediction
based on macro-level reasoning when she assumes that adding another gas to the
closed system will increase the internal pressure and shift the equilibrium in
accordance with LeChatelier's Principle. Only after observing how the molecules
interacted on the submicro-level were Mary and Kathy able to generate correct
predictions about the behavior of the system, as were the other participants as
evidenced in previous excerpts. Moreover, Kathy explicitly critiqued reasoning
at the macro-level by suggesting that a caveat to LeChatelier's Principle was
in order given her observations in Connected Chemistry.
As
with other inquiry-based modeling packages (Kozma et al., 1996), Connected
Chemistry facilitated the students' ability to link multiple representations
and levels in order to gain a deeper understanding during the interview. Connected
Chemistry moves a step beyond this linking when used in the classroom. As
before, we saw our participants develop their understandings by connecting
Connected Chemistry's concentration plots (macro-level) to the visualizations
in the graphics window (submicro-level). In the classroom, students have the
opportunity to view the procedures and information windows of Connected
Chemistry simultaneously to enhance their conception of the symbolic level and
connect it with the molecules on the submicro-level and the macro-level
concentrations. Comparing the chemical symbols of the information window with
the statements in the procedures window can help students understand how an
external factor affects a chemical reaction and how that effect is realized
mathematically in equilibrium expressions as seen in textbooks.
This article introduced a novel modeling package,
Connected Chemistry, and aimed to identify possible ways in which the learning
environment might help improve student understanding and application of
chemistry concepts, particularly chemical equilibrium. Connected Chemistry,
developed in the StarLogoT (Wilensky, 1997) and NetLogo (Wilensky, 1999b)
multi-agent modeling languages, relies on modeling chemistry concepts as
emergent phenomena to provide an inquiry-based learning environment that
emphasizes conceptual reasoning about chemistry problems. We observed several
common misconceptions about chemical equilibrium and LeChatelier's Principle in
six advanced science undergraduates who we interviewed. Each student relied on
procedures and facts when responding to pen-and-paper questions, which
frequently resulted in incorrect or incomplete answers. Over the course of
their interaction with Connected Chemistry each student came to depend less on
algorithms and rote facts and depend more on conceptual approaches to problem
solving and answer justification. Students showed the greatest improvements
with Connected Chemistry while attempting to (1) define chemical equilibrium,
(2) characterize factors that affect equilibrium, and (3) transition between
submicro-, macro- and symbolic levels during problem solving.
This study indicates that Connected Chemistry holds
promise for promoting the necessary conceptual reasoning to succeed in chemistry.
Our projected use for Connected Chemistry models in the traditional chemistry
classroom is more varied than its role in the present study. We are currently
engaged in several larger studies of learning with Connected Chemistry in high
school settings. We intend to do further study of its use and impact in several
high school chemistry classrooms and undergraduate general chemistry
laboratories. Connected Chemistry can play several roles in the classroom -- including that of a demonstration
tool, a laboratory simulator, and a student visualization and feedback tool.
The practicality and benefits of each of these methods is an additional goal of
future research. In particular, we hope to assess the impact of Connected
Chemistry on students who have the opportunity to interact with the procedural
code behind each model to determine the potential benefits of teaching
chemistry in the context of computer model design and refinement.
Model-based inquiry learning environments such as
Connected Chemistry are vital to improving student understanding in chemistry
as well as other physical sciences. The paucity of conceptual and model-based
reasoning and the ubiquity of rote memorization have resulted in students who
lack the ability to solve problems that they themselves characterize as
"basic". By presenting concepts at multiple levels using multiple
representations and providing students the opportunity for guided exploration
with instant feedback, learning environments such as Connected Chemistry have
the potential to revitalize student interest in chemistry and improve their
understanding.
Banerjee, A. C.
(1995). Teaching chemical-equilibrium
and thermodynamics in undergraduate general-chemistry classes. Journal of
Chemical Education, 72, 879-881.
Banerjee, A. C. (1991).
Misconceptions of students and teachers in chemical equilibrium. International
Journal of Science Education, 13, 487-494.
Barowy,
W., and Roberts, N. (1999). Modeling as inquiry activity in school science:
What's the point? In N. Roberts, W.
Feurzeig, & B. Hunter (Eds.), Modeling and simulation in science and
mathematics education (pp. 197-225).
Berlin: Springer Verlag.
Bodner,
G. M. (1992). Why changing the curriculum
may not be enough. Journal of Chemical Education, 69, 186-190.
Centola, D., Wilensky, U.,
& McKenzie, E. (2000). A hands-on modeling approach to evolution: Learning
about the evolution of cooperation and altruism through multi-agent modeling -
The EACH Project. In B. Fishman & S. O'Connor-Divelbiss (Eds.), Proceedings
of the Fourth International Conference of the Learning Sciences (pp. 166-173). Mahwah, NJ: Erlbaum.
Garnett, P. J., & Kenneth, T.
(1988). Teaching for understanding: exemplary practice in high school
chemistry. Journal of Research in Science Teaching, 26, 1-14.
Johnstone, A. H. (1993). The
development of chemistry teaching. Journal of Chemical Education, 70,
701-705.
Kozma, R. & Russell, J.
(1997). Multimedia and understanding: Expert and novice responses to different
representations of chemical phenomena. Journal of Research in Science
Teaching, 43, 949-968.
Kozma, R., Russell, J.,
Johnston, J., & Dershimer, C. (1990, April). College students'
understanding of chemical equilibrium. A paper presented at the annual meeting
of the American Educational Researcher Association, Boston, MA.
Kozma, R.,
Russell, J., Jones, T., Marx, N., & Davis, J. (1996). The use of
multiple, linked representations to facilitate science understanding. In S.
Vosniadou, R. Glaser, E. De Corte, & H. Mandl (Eds.), International
perspectives on the psychological foundations of technology-based learning
environments (pp. 41-60).
Hillsdale, NJ: Erlbaum.
Nakhleh,
M. B. (1992). Why some students don't
learn chemistry. Journal of Chemical Education, 69,
191-196.
Qu’lez-Pardo, J., &
Solaz-PortolŽs, J. J. (1995). Students' and teachers' misapplication of
LeChatelier's principle: Implications for the teaching of chemical equilibrium.
Journal of Research in Science Teaching, 32, 939-957.
Thomas, P. L., Schwarz, R. W.
(1998). College physical chemistry students' conceptions of equilibrium and
fundamental thermodynamics. Journal of Research in Science Teaching, 35,
1151-1160.
Tyson, L., & Treagust, D. F. (1999).
The complexity of teaching and learning chemical equilibrium. Journal of
Chemical Education, 76, 554-558.
Van Driel, J. H., de Vos W.,
& Verloop N. (1999). Introducing dynamic equilibrium as an explanatory
model. Journal of Chemical Education,
76, 559-561.
Voska, K. W., & Heikkinen,
H. W. (2000). Identification and analysis of student conceptions used to solve
chemical equilibrium problems. Journal of Research Science Teaching, 37,
160-176.
Wilensky,
U. (2001). Modeling nature's emergent patterns with multi-agent languages. In
Sendova & E. Neuwirth (Eds.), Proceedings of the eighth European Logo
conference (pp.
43-54). Linz, Austria: Virtech Ltd.
Wilensky, U. (2000, Winter).
Modeling emergent phenomena with StarLogoT. @CONCORD.org, 4, 6-10.
Wilensky, U. (1999a). GasLab:
an extensible modeling toolkit for exploring micro- and macro-views of gases.
In N. Roberts, W. Feurzeig, & B. Hunter (Eds.), Modeling and simulation
in science and mathematics education
(pp. 151-178). Berlin: Springer Verlag.
Wilensky, U. (1999b). NetLogo
[Computer software]. Evanston, IL: Center for Connected Learning and Computer
Based Modeling, Northwestern University. Available at: http://ccl.northwestern.edu/netlogo
Wilensky,
U. (1997). StarLogoT [Computer software]. Evanston, IL: Center for Connected
Learning and Computer Based Modeling, Northwestern University. Available at:
http://ccl.northwestern.edu/cm
Wilensky, U., Hazzard, E.,
& Froemke, R. (1999). An extensible modeling toolkit for exploring
statistical mechanics. In R. Nikolov, E. Sendova, I. Nikolova, & I.
Derzhanski (Eds.), Proceedings of the seventh European logo conference (pp. 377-388). Sofia, Bulgaria: Virtech Ltd.
Wilensky, U., & Reisman,
K. (1999). ConnectedScience: Learning biology through constructing and testing
computational theories--an embodied modeling approach. International Journal
of Complex Systems, 234, 1-12.
Wilensky, U., & Reisman, K. (in press). Thinking like a wolf, a
sheep or a firefly: Learning biology through constructing and testing
computational theories. Cognition & Instruction.
Wilensky,
U. & Resnick, M. (1999). Thinking in levels: A dynamic systems perspective
to making sense of the world. Journal of Science Education and Technology, 8,
3-18.
Wu,
H.-k, Krajcik, J. S., & Soloway, E. (2001). Promoting understanding of
chemical representations: Students' use of a visualization tool in the
classroom. Journal of Research in Science Teaching, 38, 821-842.
Acknowledgements
The preparation of this paper was supported by
the National Science Foundation (Grants REC-9632612 and REC-9552950). The ideas expressed here do not necessarily reflect
the positions of the supporting agency. We thank Sharona Levy, Lorenzo Pesce
and Seth Tisue, as well as two anonymous reviewers, for stimulating discussions
and critiques of our simulations and this manuscript.
APPENDIX I:
PROTOTYPICAL INTERVIEW PROTOCOL AND QUESTIONS
Protocol Segment 1: Personal Conception of Chemical Equilibrium
1. Think aloud on concept of Chemical Equilibrium
á
In your own words, can
you describe for me, what chemical equilibrium means?
á
Is it just a definition
or do you have a picture in your head of what that means?
á
Why do reactions
establish equilibrium?
á
When a reaction is at
chemical equilibrium, is the reaction changing in any way?
á
Are all chemical
reactions equilibrium reactions?
á
Do you know what factors
determine chemical equilibrium in a reaction?
2. Radioactive CH3I Problem
á
If we were to leave this
beaker, which contains radioactive CH3I on one side of an
impermeable membrane and non-radioactive CH3I on the other side, on
the counter top over night safely capped, and return the next day, would we
observe any change in the composition of each solution even though the liquid
levels have not changed?
á
What caused this change?
á
Would there be the same
amount of radioactive material in one side that there is in the other? Why or
why not?
á
Would the amount of
radioactivity on both sides be constant?
á
Is this system at
equilibrium? How did it get to equilibrium? Instantly?
3. Radioactive NaI Problem
á
If we were to leave this
beaker, which contains solid
radioactive NaI that is covered by a layer of water saturated with non-radioactive NaI on the counter over night
safely capped, and return the next day, would we observe any change in the
composition of the water?
á
What caused this change?
á
Would there be equal
amounts of solid and dissolved radioactive NaI? What if we left it for a very
long time?
á
Would the amount of
radioactivity in both states be constant?
á
Is this system at
equilibrium? How did it get to equilibrium? Instantly?
4. Review of LeChatelier's Principle
á
Have you heard of
LeChatelier's Principle (if needed)?
á
Can you tell me what
LeChatlier's Principle says?
á
Do you know all of the
factors that are included in LeChatelier's Principle?
á
If I decrease the volume
of a container that encloses an equilibrium reaction, is there any change?
á
If I add more products
to an equilibrium reaction, is there any change?
á
If I add an inert gas to
an enclosed equilibrium reaction so that I increase the pressure of the system,
how will this affect the reaction?
á
If I add a catalyst to
an equilibrium reaction, how will this affect the reaction?
á
Give Handout of
LeChatelier's Principle Review
á
Can you tell me why
decreasing volume has this effect? Is this related to pressure?
á
Can you tell me why
changing the concentration of a species has this effect?
á
Do any of the above
stressors change the rate of the reaction? The rate constants?
á
What effect does
temperature have on the system?
á
Do each of these
stressors affect the system in the same way?
Protocol Segment 2: Traditional Problem Solving
5. Symbolic description and mathematical expressions
of chemical equilibrium
2HF (l) <---> H2 (g) + F2
(g)
á
Can you tell me if this
reaction is an equilibrium reaction? How can you tell?
á
Is the rate of the
forward reaction equal to that of the reverse reaction?
á
Are the rate constants for
the reaction equal an opposite?
6. Understanding Circumstance that Affect Equilibrium
(1.) Ag2CO3 (aq) + 2HCl
(aq) --- 2AgCl (s) + H2CO3
(aq)
á
Can you tell me if the
above reaction is an equilibrium reaction?
á
How can you tell?
á
If we start this reaction
at equilibrium, will it stay at equilibrium? Why or why not?
á
Do the states of each of
these substances have any bearing on the equilibrium?
á
How about the rate? Rate
constants? Can you write the Rate Law?
(2.) HCl (aq) + NaH (aq) <---> H2
(g) + NaCl (aq)
á
Can you tell me if the
above reaction is an equilibrium reaction?
á
How can you tell?
[If needed, prompt them and tell them that we start
out at equilibrium]
á
If we start this
reaction at equilibrium, will it stay at equilibrium? Why or why not?
á
Do the states of each of
these substances have any bearing on the equilibrium?
á
How about the rate? Rate
constants? Can you write the Rate Law?
(3.) CaCO3(s) <---> CaO(s) + CO2(g)
á
Can you tell me if the
above reaction is an equilibrium reaction?
á
How can you tell?
[If needed, prompt them and tell them that we start
out at equilibrium]
á
If we start this
reaction at equilibrium, will it stay at equilibrium? Why or why not?
á
Do the states of each of
these substance shave any bearing on the equilibrium?
á
How about the rate? Rate
constants? Can you write the equilibrium expression?
á
Can you explain why Keq
= [CO2]?
7. Expected results of Chemical Equilibrium
(4.) 2NO2 (g) <---> N2O4
(g)
á
Let's say we have this
reaction and it is at an equilibrium state in a closed container?
á
Are the forward and
reverse rate constants equal? The rates themselves?
á
What happens if I shrink
the volume of the container and increase the pressure?
á
What happens if I had
more N2O4 gas to the mixture? Does the volume of NO2
change, return to what it was?
á
How about if I increase
the temperature of the system from 100 to 300 Celsius?
Protocol Segment 3: Using Connected Chemistry
8. Task completion
á
After I explain how the
model works, I would like you to complete some tasks regarding the following
reaction and tell me what you are thinking while you are solving them.
(5.) 2NO2 (g) <---> N2O4
(g)
á
Can you decrease the
volume of the container to as small as you can and describe what happens to the
system? Why did this happen?
á
Can you set Kb to 5 and
Ku to 1 and observe the reaction? What happened?
á
If we add more greens to
the system, will the system change at all? How?
á
Can we get the ratio of
reactants to products equal to 2:1? What variables would you change?
á
What would happen if we
added a catalyst to the system?
á
What would happen if we
increase the pressure?
APPENDIX 11:
SUMMARY OF LECHATELIER'S PRINCIPLE
Le
Chatelier's principle states that when a system in chemical equilibrium is
disturbed by a change of temperature, pressure, or a concentration, the system
shifts in equilibrium composition in a way that tends to counteract this change
of variable. Every change of one of the factors of an equilibrium occasions a
rearrangement of the system in such a direction that the factor in question
experiences a change in a sense opposite to the original change.
To
summarize what has been said above:
á
Decreased VOLUME of
container favors the process producing LEAST moles of total gas
á
Increased VOLUME of
container favors process producing MOST moles of gas
á
Increasing TEMPERATURE
favors the process that CONSUMES energy
á
Decreasing TEMPERATURE
favors process that PRODUCES energy
á
Increasing REACTANTS
favors the reaction that DECREASES reactant concentration
á (the same is true of products)
á
Decreasing REACTANTS
favors the reaction that INCREASES reactant concentration
á (the same is true of products)
FIGURE CAPTIONS
Figure 1. The Connected Chemistry interface window
contains a graphics display, plot output and system variables.
Figure 2. The information
window of each Connected Chemistry model contains a summary of the chemistry
concepts highlighted by the model as well as instruction on how to use the
model and suggestions on how to manipulate the NetLogo code to further explore
the simulated chemical system.
Figure
3. In the procedures window, each Connected Chemistry model provides students
access to the code that drives the simulation. The programming language of
Connected Chemistry is extremely user-friendly and annotations in the procedure
window explicitly explain the purpose of each procedure to students.
TABLES
Table I. Format of Protocol Questions
|
Interview Segment |
Example Questions |
|
Recall and Explain Chemical Equilibrium |
"Define chemical equilibrium" "State factors that can affect reaction equilibrium |
|
Traditional Problem Solving |
Ag2CO3(aq) +
2HCl(aq)<--->2AgCl(s) + H2CO3(aq) Do the states of each of these substances have any
bearing on the equilibrium position of the reaction? |
|
Problem Solving in Connected Chemistry |
Chemical Equilibrium 1 models 2NO2 (g) <---> N2O4
(g) Can you tell by looking at the graphics window only
if the system is in equilibrium?" "What else would you need to know?" "What will happen if you decrease the volume of the
container?ÉYou decreased it, what happened to the system? Is this what you
predicted?" |